

EFFICACY
If it’s COVID, choose PAXLOVID
On this page:

PAXLOVID demonstrated efficacy in the EPIC-HR trial1,2 *
EPIC-HR was a phase 2/3, randomized, double-blind, placebo-controlled trial in nonhospitalized, symptomatic adults with SARS-CoV-2 infection and ≥1 risk factor for progression to severe disease (N=2113)1
*Paxlovid has not been approved but has been authorized for emergency use by FDA under an Emergency Use Authorization (EUA) for the treatment of mild-to-moderate COVID‑19 in pediatric patients (12 years of age and older weighing at least 40 kg) who are at high risk for progression to severe COVID‑19, including hospitalization or death.
Primary efficacy endpoint: The proportion of participants with COVID-19–related hospitalization or death from any cause through day 28
COVID-19–related hospitalization or death from any cause through day 28 in nonhospitalized adults with COVID-19 (mITT1)1,2


Analysis from the mITT1 (modified intent-to-treat) analysis set (all treated participants with onset of symptoms within 5 days who at baseline did not receive nor were expected to receive COVID-19 therapeutic mAb treatment).1
For patients in the mITT analysis set2
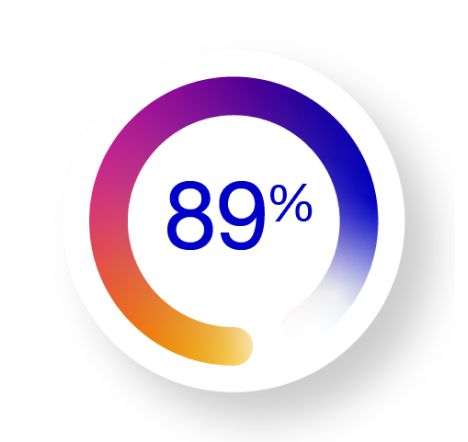
Relative measure
Relative risk reduction in COVID-19–related hospitalization or death from any cause compared with placebo
Absolute measure
- Event rates were 5/671 (0.7%) in the PAXLOVID group and 44/647 (6.8%) in the placebo group
- -6.1% difference compared with placebo (95% Cl, -8.2 to -4.1; P<0.0001)
Relative measure
An 85% relative risk reduction was observed in the mITT2 analysis set, which consisted of all treated subjects, including those who received COVID-19 therapeutic mAb treatment.2
Absolute measure
- Event rates were 10/1038 (1.0%) in the PAXLOVID group and 66/1053 (6.3%) in the placebo group2
- -5.4% difference compared with placebo (95% CI, -7.0 to -3.8; P<0.0001)

Efficacy across baseline characteristics was observed, regardless of1:
- Age
- Symptom onset
- Gender
- BMI
- Baseline SARS-CoV-2 serology status
Eligible subjects were 18 years of age and older.
What would you like to learn more about?
Drug Interactions
View boxed warning, contraindicated drugs, and other potentially significant drug interactions.
Dosing
Learn about the standard, reduced, and severe renal impairment doses of PAXLOVID.
Safety
Find out about the safety data from clinical studies and post-authorization experience and how to report an adverse event.
BMI=body mass index; CI=confidence interval; COVID-19=coronavirus disease 2019; mAb=monoclonal antibody; SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
References:
- Fact Sheet for Healthcare Providers: Emergency Use Authorization for PAXLOVID®. Pfizer Inc.; 2025.
- Data on File. CSR. Pfizer Inc.; 2023.